The advent of The Affordable Care Act (Obamacare) is on the horizon as the healthcare sector of the market continues its strong rally. With new Food and Drug Administration (FDA) regulatory easing, congressional bills, and The Obama Administration directive in 2011, 2012 saw 39 new drugs approved by the FDA, the highest number since 1996 and 30% more than were approved the previous year. The biopharma segment is the place to be moving forward, and one such company is poised to take advantage of the rally.
AVEO Pharma (AVEO) engages in discovering, developing, and commercializing targeted cancer therapies using its Human Response Platform. Compared to traditional methods, this advanced platform allows AVEO Pharma to more accurately replicate populations of tumors and cancerous mutations akin to those seen in human populations. Its principal product candidate is tivzanib, indicated for the treatment of patients with advanced renal cell carcinoma (RCC). The company is also engaged in a Phase II clinical trial for the first-line therapy in patients with advanced metastatic colorectal cancer, and a Phase II clinical trial for the treatment of patients with locally recurrent or metastatic triple negative breast cancer.
- Upcoming catalyst
An FDA Advisory committee will convene on May 2nd to vote for or against recommending tivzanib for approval.
tivozanib is an oral, once-daily, investigational tyrosine kinase inhibitor. AVEO has submitted a New Drug Application (NDA) that is currently under review by the U.S. Food and Drug Administration (FDA) seeking approval for tivozanib in patients with advanced renal cell carcinoma (RCC), otherwise known as Kidney Cancer. tivozanib is a selective and long half-life inhibitor of all three vascular endothelial growth factor (VEGF) receptors that is designed to optimize VEGF blockade while minimizing off-target toxicities.
In January of this year, the company reported positive Phase III results from a study of 517 patients with RCC. The study compared tivozanib to a current frontline treatment for RCC, sorafenib. Sorafenib is marketed by Onyx Pharmaceuticals (ONXX) under the trade name of Nexavar - global Nexavar net sales topped over $1 billion in 2011.
Dose reductions of the drugs compared were 11.6% with tivozanib versus 42.8% with sorafenib (P<.001). Discontinuations occurred in 4.2% of the tivozanib arm versus 5.4% in comparison to the Nexavar arm. Overall, there were fewer drug-related adverse events with tivozanib at 67.6% in comparison to Nexavar at 83.3%.
Tivozanib was especially useful in treatment-naive patients, who comprised 70% of study population. In those patients, progression-free survival was 12.7 months with tivzanib and 9.1 months with Nexavar (HR, 0.756; P = .037).
"This is the first phase 3 trial to show greater than 12 months progression-free survival in treatment-naïve patients (with advanced renal cell carcinoma)," said Dr. Eisen, a tivozanib-1 investigator from the University of Cambridge in the United Kingdom.
Shares of AVEO dropped when the final overall survival data (OS) was announced February 13th of this year. Investors were initially underwhelmed by tivozanib's OS of 28.8 months versus 29.3 months for Nexavar. However, the study had a one-way crossover allowance, which permitted patients to switch therapies if the disease progressed or their symptoms worsened. So, why did tivozanib have a lower OS?
In the TARGET study where Nexavar was compared to Placebo, Nexavar showed a 6 months post-crossover OS of 19.3 months (data presented at 2006 ASCO). As mentioned above, in AVEO's Phase III study of tivozanib vs Nexavar, Nexavar showed an OS of 29.3 months. Why is there a 10 month improvement in Nexavar's OS between the two studies?
Because AVEO's study allowed up to 67% (by 26 months) of Nexavar's arm to cross over to receive tivozanib treatment, whereas patients in the tivozanib arm had minimal crossover (10%) into the Nexavar treatment, there is strong reason to believe that the crossover to tivozanib treatment contributed to the bulk of Nexavar's 10 additional months of OS benefit with dual treatment.
Dr. Eisen first indicated that OS with tivzanib was not a measurement of efficacy, and probably could not be, since patients who progressed on Nexavarwere given the option of crossing over to tivzanib, which would compromise any overall survival figure for this arm of the study.
This same phenomenon in divergence between OS rates has occurred with other drug trials before. For example, when Dendreon's (DNDN) Provenge was prescribed first, then Zytiga afterwards, OS rates went up, but Zytiga on its own failed to show any improvement in OS. In other words, when doctors prescribed Zytiga first, they put their patients at risk of having the disease progress.
The rate of dose interruptions due to adverse events was 18% for tivzanib compared to 35% for Nexavar (p<0.001). The rate of dose reductions was 14% for tivzanib compared to 44% for Nexavar (p<0.001). This is a strong sign of patient tolerability, which leads to better patient compliance.
Further data shows that 74% of the patients who switched to tivzanib had evidence of tumor regression and overall confirmed response rate was 13% - a clear sign of strong efficacy.
Patients who started with Nexavar and switched to tivzanib after disease progression achieved a median progression‐free survival of 8.4 months and a median overall survival of 19.6 months. These numbers are among the best seen for patients treated in the second‐line setting and a strong reason why the FDA committee will recommend tivzanib for approval.
If tivzanib gains FDA approval, (which I strongly believe it will) through its partnership with Astellas, AVEO has the potential to receive up to $1.3 billion in milestones for RCC alone - an extremely important value driver for the company and its shareholders.
While the upcoming catalyst is very important for the company, it's apparent to me that investors are too single focused on tivzanib treating RCC, and whether or not the drug will be a front line or second line treatment for the disease. What is more important here is the company's overall platform monetary potential. As mentioned above, tivzanib is being studied in triple negative breast cancer, which is a huge untapped market. tivzanib and the platform it's based on has shown better patient tolerability, which results in better patient compliance.
One of the goals of Obamacare is to help increase patient compliance and AVEO's platform approach provides just that. When compared to its peers, tivzanib's side effect profile is markedly superior.
Approval of tivzanib opens the door for AVEO to further advance studies for tivzanib and other pipelined drugs because they will receive up to $1.3B from Astellas. Also, another important benefit from tivzanib gaining approval for investors is that the company should not have to engage in an ymore finance deals, so shareholder dilution should not be an issue moving forward. Astellas milestone payments should have this covered.
In part, this is the same bullish thesis I have been making for Arena Pharma (ARNA) since Belviq was approved last year -- Belviq was the first anti-obesity drug approved by the FDA since the late 1990s.
There has been great debate between the bulls and bears concerning the revenue potential of Belviq. Some believe the drug will bring in massive revenues, while others think differently. Even if Belviq falls short of bullish expectations, revenue from the drug should provide the company the needed capital to advance its other pipelined drugs without having to accept less-than-favorable royalty deals. The company should no longer have to dilute investors with market based capital raises. This should provide Arena with the needed leverage to grow the company substantially larger over the next few years, regardless of whether Belviq is a huge winner or not.
Another company with a similar situation to AVEO is Ariad Pharma (ARIA).
Merck (MRK) entered into a milestone payment agreement with Aria for ridaforolimus in July of 2007. Merck made a $75 million upfront payment to ARIAD and after that paid ARIAD $53.5 million in milestone payments for the initiation of Phase II and Phase III clinical trials of ridaforolimus, in addition to paying a 50 percent share of ridaforolimus development, manufacturing and commercialization costs.
Ridaforolimus is an investigational targeted and small-molecule inhibitor designed to treat sarcomas. In June of 2012, the FDA rejected Ariad and Merck's New Drug Application (NDA) by issuing a complete response letter (CRL) regarding the drug.
However, because Ariad received a good amount of money from Merck for ridaforolimus, the company was able to use the capital to help develop Iclusig™ (ponatinib), which was approved by the FDA late last year. Ponatinib is a BCR-ABL inhibitor designed to treat adults with chronic myeloid leukemia (CML) and Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ ALL), two rare blood and bone marrow diseases. As late as 2009, Ariad stock was selling under $0.80 a share. Based on the speculation surrounding ponatinib, the stock traded as high as $25 a share in late 2012.
The most important point that I feel investors need to consider is the potential of AVEO's entire platform, rather than merely focusing on whether or not tivzanib will be approved as a first or second line RCC treatment. As previously mentioned, the company stands to receive $1.3B from Astellas in milestones upon FDA approval of tivzanib. Additionally, the $1.3B in milestones are only for the RCC approval segment, where potential sales could reach $400M. Why would Astellas be willing to pay so much for peak sales in RCC of $400M?
Part of the answer might be found in the fact that AVEO is currently engaged in its BATON‐BC Phase II trial, which is designed to evaluate tivzanib in patients with triple‐negative breast cancer. Triple negative breast cancer is a lucrative market because there are currently no targeted therapies approved for this segment.
Tivzanib's front line potential to treat such diseases as triple negative breast cancer and colorectal cancer is likely the reason Astellas is willing to pay AVEO over $1B in milestones. A fellow Seeking Alpha contributor mentioned AVEO the other day, and gives the company an evaluation of $3B by 2022, but solely based on tivozanib treating RCC. He seems to have overlooked BATON-BC and the company's desire to study the drug for colorectal cancer. This makes my point of investors being so single focused on tivozanib to solely treat RCC, (and the OS debate) that they are not doing the deeper due diligence and focusing on the much larger potential for AVEO's pipeline to treat an array of diseases with large lucrative market potential.
AVEO's early clinical pipelined drug AV-203 would see a huge benefit if tivzanib gains FDA approval. AV-203 is a monoclonal antibody that selectively targets the receptor ERBB3, a new and promising strategy for treating various cancers.
In March 2009, AVEO announced an exclusive option and license agreement with Biogen Idec, Inc. (BIIB) for the development and commercialization of ERBB3-targeted antibodies. Through the agreement, AVEO retains North American commercialization rights to AV-203 and all ERBB3-binding antibodies, and is responsible for leading global development of the ERBB3 program.
In May 2012, AVEO announced the initiation of a Phase 1 study examining the safety and preliminary efficacy of AV-203 along with exploratory biomarkers in patients with advanced solid tumors. AV-203 was developed through AVEO's Human Response Platform™, which evaluates drugs that can block the function of cancer-causing target genes and identifies biomarkers that are indicators of drug response or resistance in patients.
Recent Insider Buys
| Date | Insider | Shares | Type | Transaction | Value* |
| Mar 6, 2013 | TERMEER HENRI A Director | 55,499 | Direct | Purchase at $6.73 per share. | 373,508 |
| Feb 18, 2013 | YOUNG ROBERT C Director | 990 | Direct | Purchase at $6.60 per share. | 6,534 |
Director Termeer seems rather bullish on AVEO, as evident from his 55,499 direct purchase.
| Share Statistics | |
| Avg Vol (3 month): | 771,318 |
| Avg Vol (10 day): | 661,950 |
| Shares Outstanding: | 43.53M |
| Float: | 36.45M |
| % Held by Insiders: | 5.28% |
| % Held by Institutions: | 71.60% |
| Shares Short (as of Mar 28, 2013): | 4.64M |
| Short Ratio (as of Mar 28, 2013): | 8.00 |
Out of these share statistics, it is important to note the 4.64 million shares held short. With the FDA Adcomm quickly approaching, shorts covering their position should lead to stock price appreciation. I would not expect the shorts to risk substantial losses, as there is a very high chance we see a positive result from the Adcomm.
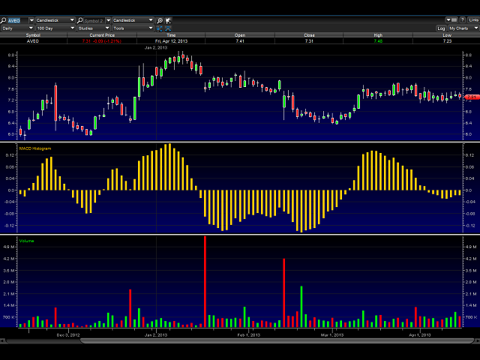
The stock price has been consolidating nicely here between $7.25 and $7.70. The MACD histogram looks like it wants to cross over positive, and it also appears shorts have been churning out of their positions.
The chart looks set up going into the May 2nd Adcomm for a run to over $8.50.
Conclusion:
AVEO has a market cap of $318.23M, which in my opinion, is about $180M too low when considering the greater potential of tivzanib as a front line drug to treat diseases such as triple negative breast cancer among others. AV-203 has begun a Phase II study that could lead to a pivotal Phase III study, and we have an FDA Adcomm quickly approaching. I predict the Adcomm will be positive, and that tivzanib will win FDA approval for RCC. As mentioned prior, it is not of utmost importance whether tivzanib will be a first line or second line treatment. What is more important is the money AVEO will receive from Astellas to further advance tivzanib as a first line treatment for diseases that have much higher monetary potential.
Short term price target opinion heading into Adcomm is $8.50 - $9.
Price target opinion for a positive Adcomm is $11 - $11.50.
FDA approval and long term target if tivzanib is ultimately approved for other indications is $25+.
Additional disclosure: Disclaimer: This article is intended for informational and entertainment use only, and should not be construed as professional investment advice. They are my opinions only. Trading stocks is risky -- always be sure to know and understand your risk tolerance. You can incur substantial financial losses in any trade or investment. Always do your own due diligence before buying and selling any stock, and/or consult with a licensed financial adviser.