The following 5 biotech companies have strong catalysts and/or strong chart signals that indicate a trade to the long side could be in order. With the current choppy markets, opportunities for long trades are few and far between. I will give specifics for each company on why I believe they should provide a nice upside gain.
Sarepta Therapeutics, Inc. (NASDAQ: SRPT) focuses on the discovery and development of RNA-based therapeutics for the treatment of serious and life-threatening rare and infectious diseases. Its lead clinical candidate is Eteplirsen, which is in Phase 2 clinical stage for the treatment of Duchenne muscular dystrophy. The company is also developing treatments that are in Phase I clinical trials for infectious diseases, including AVI-7537 for Ebola virus; AVI-7288 for Marburg virus; and AVI-7100 for influenza. Its pre-clinical products comprise Exon 45 PMO and Exon 50 PMO, which are used for the treatment of duchenne muscular dystrophy.
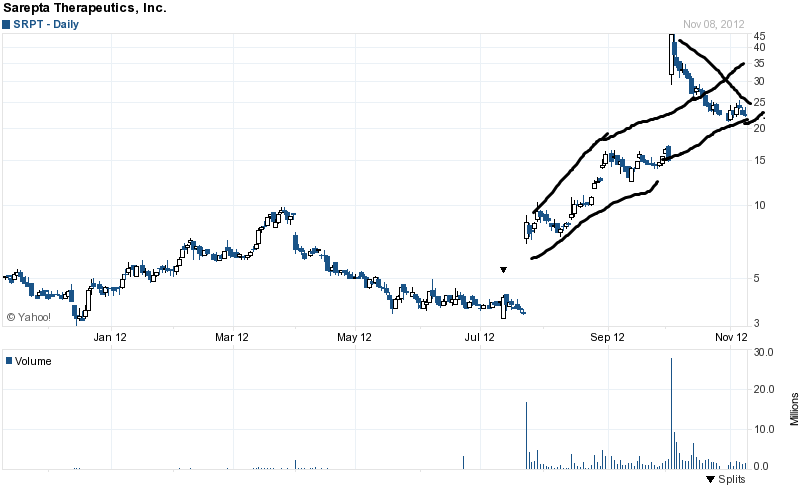
On 10/3/12, Sarepta announced that it received positive phase IIb clinical data for its Duchenne muscular dystrophy (DMD) drug eteplirsen. Sarepta said eteplirsen slowed the progress of the disease and increased patients' levels of the protein dystrophin in an extension of a midstage trial. Low levels of that protein are the cause of Duchenne muscular dystrophy. The stock exploded from a prior day's closing price of $14.99 to an intra-day high the next trading session of $45.00.
The stock has since pulled back, trading in near $22 a share currently.
DMD is one of the most common fatal genetic disorders to affect children around the world. Approximately one in every 3,500 boys worldwide is affected with DMD. Girls are rarely affected by the disorder. DMD is a devastating and incurable muscle-wasting disease associated with specific inborn errors in the gene that codes for dystrophin, a protein that plays a key structural role in muscle fiber function. Symptoms usually appear in children by age three. Progressive muscle weakness of the legs and pelvis eventually spreads to the arms, neck, and other areas. By age 10, braces may be required for walking, and most patients require full-time use of a wheelchair by age 12. Eventually, this progresses to complete paralysis and increasing difficulty in breathing due to respiratory muscle dysfunction requiring ventilatory support, and cardiac muscle dysfunction leading to heart failure. The condition is terminal, and death usually occurs before the age of 30. The outpatient cost of care for a non-ambulatory DMD patient is very high. There is currently no cure for DMD, but for the first time ever there are promising therapies in, or moving into, development. Any positive news on the advancement of this drug and Sarepta stock is back to the races - so to speak.
The chart above shows us that Sarepta has retraced back to its original uptrend line, after the huge gap up from a few weeks ago. In my opinion, we are at a pivot point for the stock, and all indications seem to point to the uptrend continuing from around its current price
BSD Medical (NASDAQ: BSDM) develops, manufactures, markets, and services systems to treat cancer and diseases using heat therapy delivered via focused radiofrequency and microwave energy. The company develops technology and products for thermal ablation and hyperthermia cancer therapy through various techniques, which include thermal ablation that ablates soft tissues at high temperatures through focused microwave energy.
The current options for cancer patients include the following:
- Surgery
- Ablation
- External Radiation
- Systemic Chemotherapy
- Embolization (chemo/radio)
With the use of BSD's ablation technology, cancer patients have the opportunity for a non-invasive alternative to open surgery. Because it is non-invasive, complications such as infection, swelling, and fluid accumulation can be avoided. BSD ablation tech can cut down on, or eliminate 4 of the above current cancer treatments.
BSD's MicroThermX Microwave Ablation System (MicroThermX) is a compact, mobile, state-of-the-art, proprietary system that includes a microwave generator, single-patient-use disposable antennas, and a thermistor-based temperature monitoring system. The innovative design of the MicroThermX is the first of its kind that allows delivery of higher power levels using a single generator. The MicroThermX utilizes innovative, patented, synchronous phased array microwave delivery technology that was developed by the Company. This microwave technology provides larger and more uniform size of ablations during a single procedure. The MicroThermX introduces into the product line an innovative disposable antenna (SynchroWave antenna). Up to three SynchroWave antennas are used in each ablation treatment, which will provide a significant ongoing revenue stream after the sale of the system. This antenna gives BSD the ability to treat smaller tumors in the liver, lung, kidney, and other areas. The soft tissue ablation world market potential is estimated to exceed $2.3 billion.
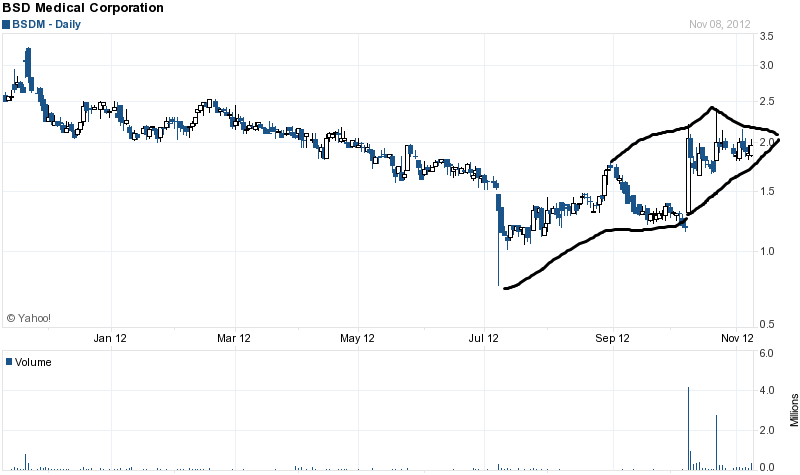
An upcoming catalyst for BSD is an earnings call scheduled for this Monday, November 12th. The company recently reported significant growth of MicroThermX microwave ablation system sales and equipment utilization. The report notes a 391% increase in sales for the MicroThermX product line for September 2012, as compared to September 2011. Because of this, I think it's likely that the earnings will be a positive surprise to investors.
Let's take a look at the chart and see what it has to say:
The chart above shows a nice pennant formation occurring with a symmetrical wedged triangle. This stock has a good chance to break above $2.50 in the very short term -- if it gets good volume.
Amicus Therapeutics, Inc. (NASDAQ: FOLD) focuses on the discovery, development, and commercialization of orally-administered, small molecule drugs for the treatment of lysosomal storage disorders and diseases of neurodegeneration. Its drugs are known as pharmacological chaperones, which selectively bind to the target protein, enhance the stability of the protein, help it fold into the three-dimensional shape, as well as allow proper trafficking of the protein. This increases protein activity, enhances cellular function, and reduces cell stress.
In its Fabry program, the company is investigating the use of AT1001 to bind to destabilized α-galactosidase A enzyme (α-GAL) and thereby restore its intended biological function.
Fabry disease is a rare X-linked (inherited) lysosomal storage disease, which can cause a wide range of systemic symptoms. It is a form of sphingolipidosis, as it involves dysfunctional metabolism of sphingolipids.
There has been historic difficulty in finding successful treatments for rare genetic diseases such as Fabry's. If Amicus can show successful phase III data here, it could very well be like a "kodak" moment in medicine's war against genetic diseases.
On September 6th, Amicus issued a press release to update the screening profiles related to this study. Labeled study 011, this is one of two ongoing Phase 3 studies of migalastat HCl monotherapy being conducted by Amicus and GlaxoSmithKline (NYSE: GSK). The updated screening profiles included the following:
- A total of 180 Fabry patients (60 males and 120 females) were screened for Study 011. Prior to screening sites could have used genotype information when available to enrich for Fabry patients with amenable mutations who were more likely to be interested in participating.
- Approximately 86% (154/180) of patients screened had missense mutations (compared to a current estimate in the Fabry population of approximately 60%).
- Approximately 88% (136/154) of those patients, or 76% of patients screened, had alpha-galactosidase A mutations amenable to migalastat HCl monotherapy, and were potentially eligible for enrollment.
- Approximately 50% (67/136) of those patients, or 37% of all patients screened, enrolled in Study 011 upon meeting all entry criteria, including: 1) naïve to ERT or had not received ERT for at least 6 months prior to study entry; 2) genetic mutations amenable to chaperone monotherapy and; 3) for study purposes, urine globotriaosylceramide (GL-3) levels at least four-times the upper limit of normal at baseline.
The primary endpoint in Study 011 is a change in interstitial capillary GL-3 as measured in kidney biopsy at 6 months versus baseline. The six month primary treatment period in Study 011 was completed in the second quarter of 2012 and the six-month follow-up period is expected to complete in the fourth quarter 2012. Amicus and GSK will also un-blind and analyze data from the primary six month treatment at this time. Currently, both companies remain blinded to all results.
Also of significance, on Nov. 8, 2012, the company announced additional preliminary results from an open-label Phase 2 drug-drug interaction study (Study 013) to evaluate a single oral dose of migalastat HCl (150 mg or 450 mg) co-administered with enzyme replacement therapy (ERT) in males with Fabry disease. In a poster at the American Society of Human Genetics (ASHG) Annual Meeting, Dr. David G. Warnock, University of Alabama-Birmingham, presented results from all 12 patients in the migalastat HCl 150 mg dose group.
Amicus, also in collaboration with GlaxoSmithKline, is developing the investigational pharmacological chaperone migalastat HCl as a monotherapy and in combination with ERT for the treatment of Fabry disease. When co-administered with ERT, migalastat HCl binds to and stabilizes infused enzyme in the circulation.
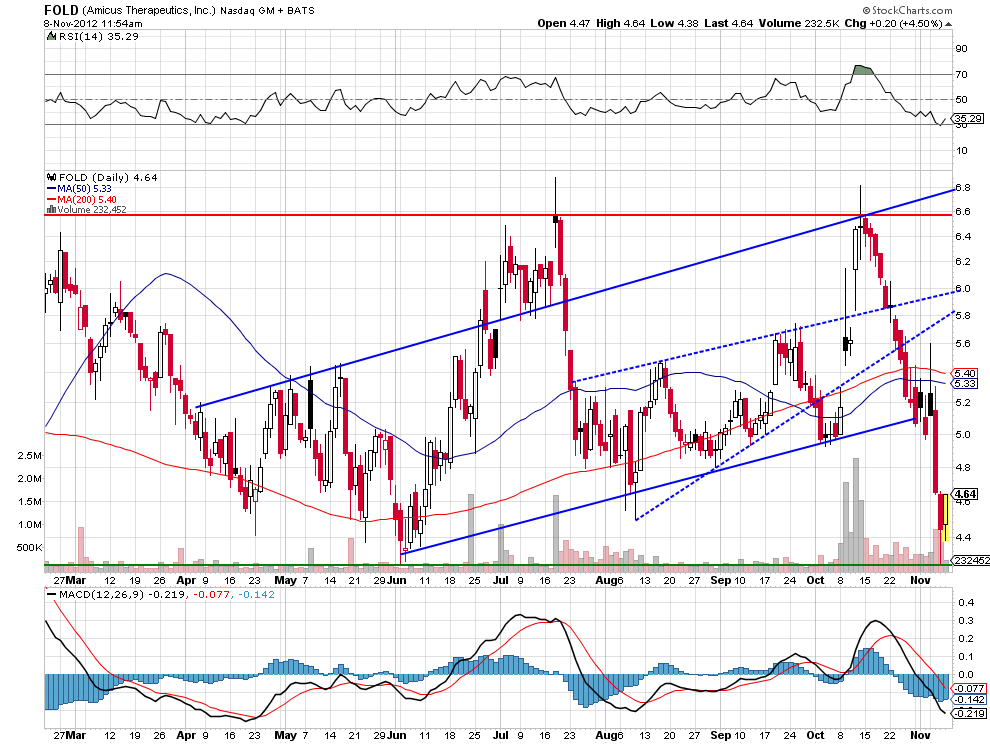
In my opinion, Amicus is grossly undervalued at its current price of $4.77 with a market cap of $235.45M. With an average cost of
$200,000 US per patient in treating a disease like Fabry's, the potential for Amicus to rake in big revenues is very high, notwithstanding GSK is likely to buyout the company on successful phase III data from the 011 study.
The chart above shows us that Amicus has been oversold, and received a nice bounce yesterday, trading as high as $4.78 before closing at $4.75. The chart seems to indicate a reversal is currently underway, and with the company already being undervalued, it's a good bet in my opinion that we will see $5 very soon.
Threshold Pharmaceuticals Inc. (NASDAQ: THLD) focuses on the discovery and development of drugs for the microenvironment of solid tumors and the bone marrows of hematologic malignancies as novel treatments for patients living with cancer. Its clinical development products include TH-302, a novel drug candidate, which is in Phase I, Phase II, and Phase III clinical trials for the severe hypoxic regions present in various solid tumors and hematologic malignancies.
TH-302 is an anticancer agent in clinical development at Threshold. Preclinically, it is preferentially activated under hypoxic conditions and has demonstrated potent anticancer activity in many preclinical cancer models. TH-302 is converted selectively to the drug's active form, dibromo isophoramide mustard, a potent DNA alkylator, within hypoxic tumor cells. TH-302 targets levels of hypoxia that are common in tumors but are rare in normal tissues - this is how selective targeting of the tumor occurs.
After conversion to the active form of the drug, the more resistant hypoxic cells are exposed to high concentrations of released cytotoxic agent, which can also diffused into the surrounding oxygenated regions of the tumor, exerting what is referred to as a bystander effect. In this way, TH-302 can kill more of the tumor than can otherwise be accounted for by the hypoxic fraction alone. Because of its selective activation in the hypoxic regions of solid tumors, it is believed that TH-302 will be less likely to produce the systemic toxicity caused by most cytotoxic chemotherapies.
At the end of 2012, Threshold and its partner Merck KGaA plan to initiate a phase III trial that will compare TH-302 in combination with gemcitabine against gemcitabine alone in patients with metastatic or locally advanced unresectable pancreatic cancer. The link above to an article written by By Mihai Ciustea, Ph.D gives a solid overview on the drug.
Others as in this linked article, have mentioned that they did not think TH-302 was all that impressive. Personally, at a price of $4.05 and a market cap of $227.82M, I think it's worth the gamble that Mihai's point of view is correct.
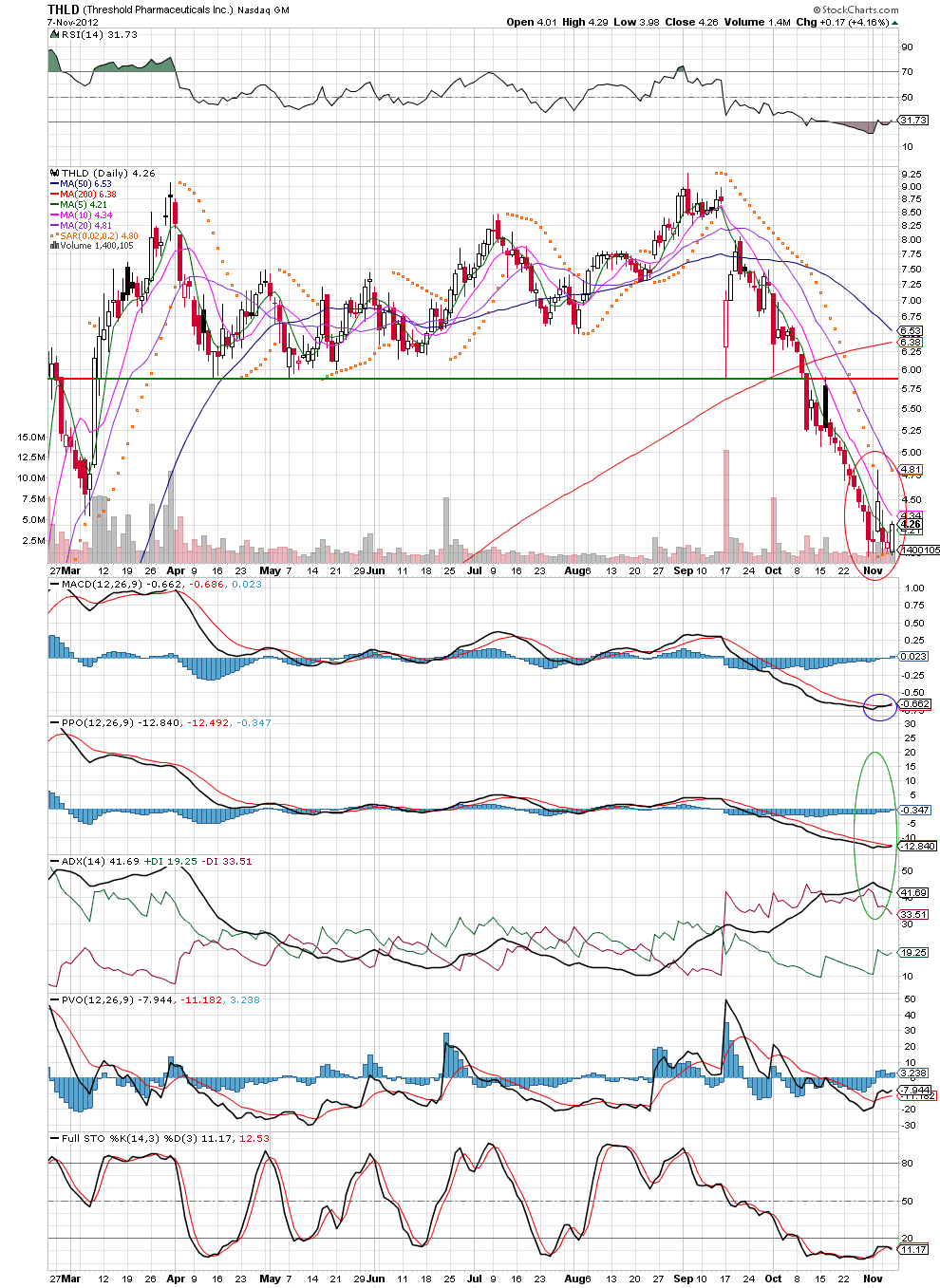
Let's take a look at the chart to see if Threshold offers a good long trade here.
As we can see from the chart above, the stock has that classic PPO/ADX pinch and, in my opinion can easily make its way back to about $5.88 per share which would be a rather significant move from here. A few more things to note, the stock is showing signs of bottoming around the 4 dollar range with a MACD cross, and yesterday crossed above the 5 day moving average (MA). Watch for a follow through and consider a stop just below the most recent bottoming level -- around $3.90
Ironwood Pharmaceuticals, Inc. (NASDAQ: IRWD) discovers, develops, and commercializes human medicines. Its lead product candidate, linaclotide, is a guanylate cyclase type-C agonist that received FDA approval in August of this year for the treatment of patients with irritable bowel syndrome with constipation (IBS-C) and chronic constipation (CC). The company also has a pipeline focused on the research and development of early development candidates and discovery research in various therapeutic areas, including gastrointestinal diseases, central nervous system disorders, respiratory diseases, and cardiovascular diseases.
Insider activity is always something I like to look at, as it tends to be bullish when key insiders buy a good amount of shares in their company. On November 5, The CEO of Ironwood, Peter Hecht, purchased 50,000 shares and currently holds 3,722,554 shares of the company. He has led Ironwood since its inception in 1998.
The day after this purchase, the company reported its third quarter 2012 financial results on November 6, which looks strong in several areas such as:
- Revenue- $96.4 million
- Net income- $47.6 million
- Cash- $193.4 million
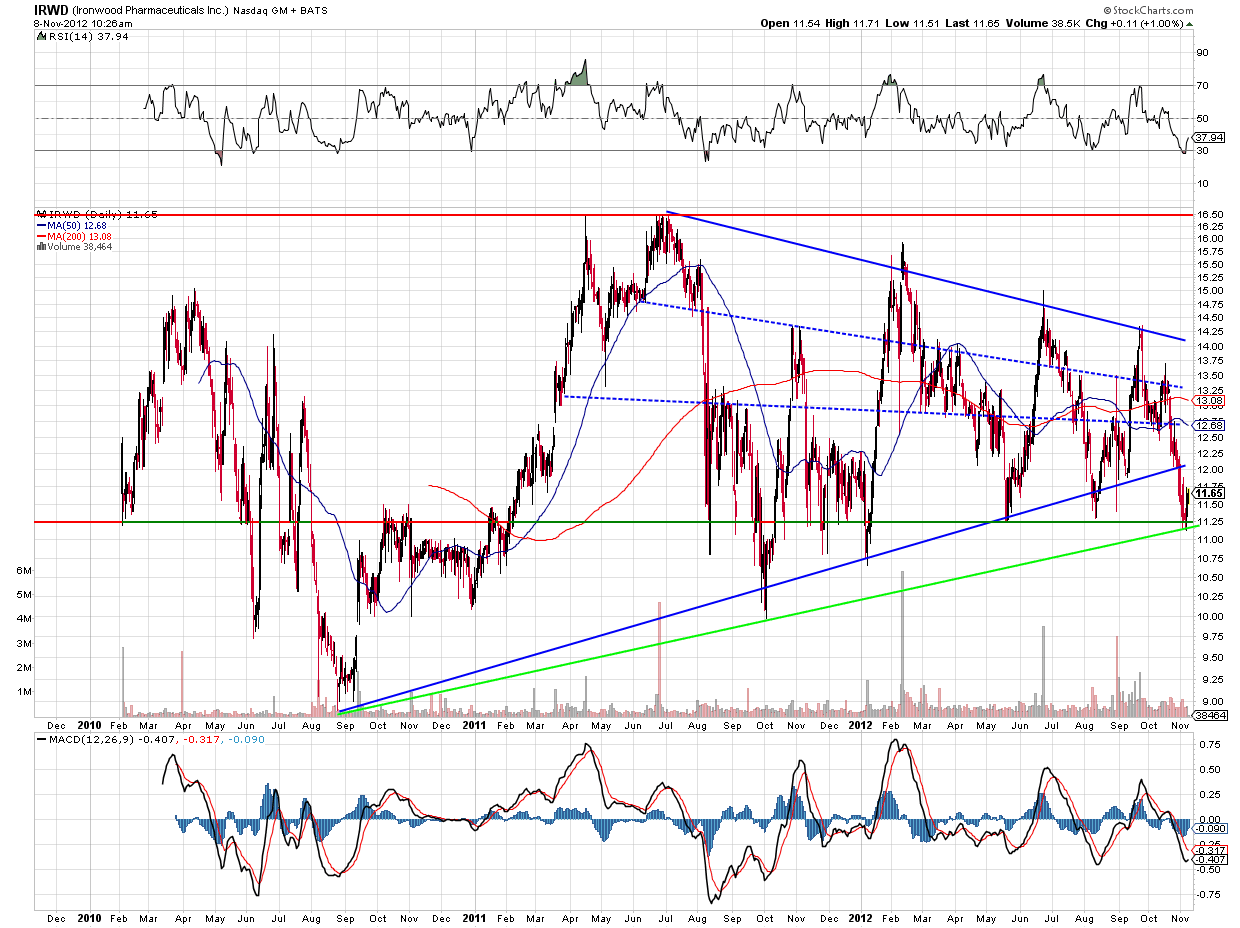
Let's take a look at the chart:
The chart above shows me a nice wedge formation taking place. Notice the higher lows and lower highs, and the amount of trading taking place above the green retrace line. The stock closed yesterday's session at $11.68, and the chart seems to indicate a move to $12.50, barring a large downturn in the overall market.
I have offered 5 companies in this write-up I feel have both strong fundamental and technical factors that could indicate these stocks are good upside trades. This is a tough stock market right now, but I feel the bottom is close to being priced in. We have the holiday season approaching fast, and usually we can expect to see a nice bounce in all the indices as we draw closer to year end.
Disclosure: I am long BSDM, FOLD. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it. I have no business relationship with any company whose stock is mentioned in this article.
Additional disclosure: Disclaimer: This article is intended for informational and entertainment use only, and should not be construed as professional investment advice. They are my opinions only. Trading stocks is risky -- always be sure to know and understand your risk tolerance. You can incur substantial financial losses in any trade or investment. Always do your own due diligence before buying and selling any stock, and/or consult with a licensed financial adviser.

Nice piece Scott, and thanks as always – first time I’ve seen you write about a device company! Speaking of which – have you had a chance to look at NVGN (which has been going nuts over the last few days)?
btw – i know market’s doing well today, but you’re batting % on these picks is insane!!!
I’m lucky 🙂
Scott, bring me up to date on your feelings re. atrs. After all, it’s because of you and a few others on the Yahoo board that I respect I purchased 9139 shares. Thank you, JOE
Scott–update on BSDM? Earnings not great–speculation by some that the sales increases you mention won’t show up until next quarter’s Earnings report in Jan?