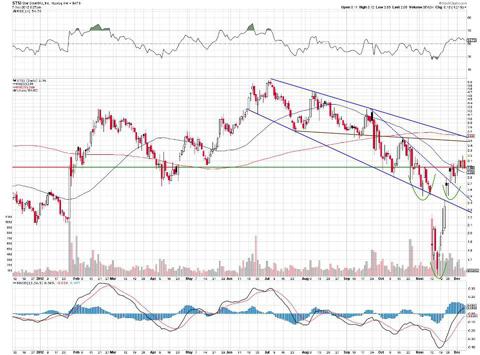
Star Scientific (STSI)
Star Scientific engages in the manufacture, distribution, and sale of consumer products, dietary supplements, and dissolvable tobacco. They also have several products which have a botanical-based component designed to treat a range of neurological conditions including Alzheimer's disease, Parkinson's disease, schizophrenia, depression, and tobacco dependence. Its products primarily include Anatabloc for anti-inflammatory support, and CigRx, a tobacco alternative. The company also develops, implements, and licenses technology behind its proprietary StarCured tobacco curing process, which prevents the formation of carcinogenic toxins present in tobacco and tobacco smoke. In addition, it is involved in the development, manufacture, marketing, and sale of less toxic and dissolvable smokeless tobacco products. These include ARIVA compressed powdered tobacco cigalett pieces, STONEWALL Hard snuff, and modified risk tobacco products.
Moving Factors: Chart and possible catalyst.
Star has received favorable FDA rulings regarding its smokeless tobacco products in the past, but it's not the tobacco products I like about the company. I'm much more interested in the compound Anatabloc, which could be used for treating chronic inflammation associated with disorders such as thyroiditis, cancer, arthritis, Alzheimer's disease, and multiple sclerosis.
Anatabloc contains the dietary ingredient anatabine citrate. Anatabine is an alkaloid that is found in a variety of plants in the Solinaceae family including the tobacco plant. Anatabine citrate, combined with Vitamins A and D3, is believed to assist the body in maintaining anti-inflammatory support. Excess inflammation is linked with a variety of auto-immune conditions that include thyroid dysfunction and arthritis. A provisional patent application has been filed with the US Patent & Trademark Office for the Anatabloc product.
Johns Hopkins University School of Medicine scientists believe they know why Star's anatabine compound Anatabloc works. John Hopkins is the location where the thyroid research and human studies have been funded by a grant from the Walton Family Foundation.
Testing and peer review has been conducted on Anatabloc, and theoretical explanations have appeared in such leading journals as Endocrinology as to why Antabloc should work in patients with thyroid dysfunction and arthritis.
Star Scientific remarked in July of this year:
The current impressive results achieved by the Johns Hopkins team strongly support the first look at the thyroid data in man, which we anticipate will be available in the third quarter.
In early October 2012, Johns Hopkins University School of Medicine was expected to release results of a human thyroid study using the dietary supplement Anatabloc. The study was being performed in nine clinical sites in Michigan, Texas, New Jersey, Illinois, and Florida.
We are now in Q4, but the data has not yet been released. I decided to look into the delay, and my anonymous sources tell me we might see positive results announced as early as this week. If the results are positive as rumored, this would take Anatabloc from "working in theory," to the human trial data showing that the compound does in fact work.
In addition to the thyroid indication, researchers at the Roskamp Institute demonstrated that anatabine can suppress brain inflammation in animal models of Alzheimer's disease, inflammation in the blood in mice, and inflammation induced in human blood once removed from the body.
The reason why this is critical is because inflammation in the brain appears to be a big reason for several diseases, most notably relevant in regards to Star Scientific's research into Alzheimer's disease. According to the National Institute of Health in Bethesda, MD, inflammation clearly occurs in pathologically vulnerable regions of the Alzheimer's disease (AD) brain, and it does so with the full complexity of local peripheral inflammatory responses. In the AD brain, damaged neurons and neurites provide obvious stimuli for inflammation. Cumulated over many years, direct and bystander damage from AD inflammatory mechanisms is likely to significantly exacerbate the very pathogenic processes that gave rise to it. Thus, animal models and clinical studies, although still in their infancy, strongly suggest that AD inflammation significantly contributes to AD progression. By better understanding AD inflammatory and immuno-regulatory processes, it should be possible to develop anti-inflammatory approaches that may slow the progression or delay the onset of this devastating disorder.
| Shares Short (as of Nov 15, 2012): | 29.99M |
| Short Ratio (as of Nov 15, 2012): | 11.10 |
| Short % of Float (as of Nov 15, 2012): | 25.20% |
| Shares Short (prior month): | 30.62M |
Considering the high short interest listed above, Star should make for a nice short term long based trade. I also consider the possible positive news regarding Star Scientific's human thyroid study a possible catalyst opportunity trade that could result in a stock price considerably higher than Friday's closing price of $2.92. Furthermore, I would expect Rock Creek Pharmaceuticals, Inc., Star's wholly owned subsidiary, to begin enrolling for its Clinical Phase II shortly thereafter.
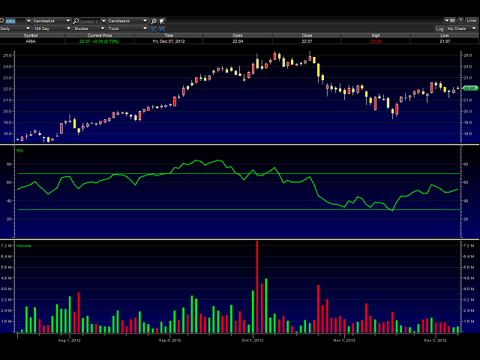
Star has been in an extended downtrend for awhile, but has recently appeared to show signs of reversal. It has formed an inverted head and shoulders pattern, and has broken two downtrend resistance lines. The stock appears to have found a base of support at the neckline area, and looks poised to make the next leg higher. The near term target price is $3.50-$3.60, and If it breaks this range on higher volume, look for a test of $5.
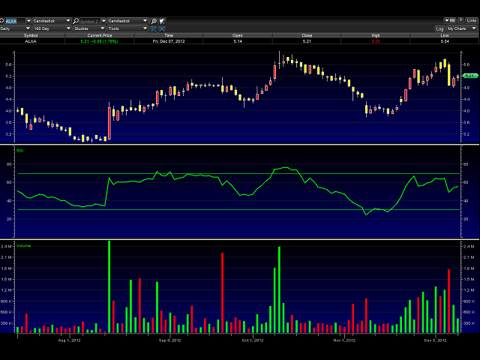
Alexza Pharma (ALXA)
Alexza engages in the research, development, and commercialization of novel proprietary products for the acute treatment of central nervous system conditions worldwide. Its product candidates are based on a proprietary technology, the Staccato system, which vaporizes an excipient-free drug to form a condensation aerosol that allows for rapid systemic drug delivery when inhaled. The company's lead product candidate is Adasuve for the acute treatment of agitation in adults with schizophrenia or bipolar disorder.
Moving factors: 2 near-term FDA catalysts, chart technicals.
Alexza has 2 significant catalyst events coming up that could move the stock strongly to the upside in the short term.
The first catalyst event for Alexza is a decision on its Marketing Authorization Application (MAA) submitted to the European Medicines Agency (EMA) for Adasuve. This decision is scheduled for the middle of December, 2012.
The second catalyst event for the company is Adasuve 's Prescription Drug User Fee Act (PDUFA) date of 12/21/12, which is the bigger catalyst driver.
In May of this year, Alexza received a Complete Response Letter (CRL) from the FDA regarding its New Drug Application (NDA) for Adasuve inhalation powder 5 mg and 10 mg.
In the CRL, the FDA noted,
During a recent inspection of the Mountain View, CA manufacturing facility for this application, our field investigator conveyed deficiencies to the representative of the facility. Satisfactory resolution of these deficiencies is required before this application may be approved.
The CRL went on to state that discussions can continue on the proposed Risk Evaluation and Mitigation Strategy (REMS) after the response to the action letter has been submitted. The CRL also contained comments on Alexza's draft product labeling. The company now believes there is a substantial agreement with the FDA on the REMS and product labeling.
Senior Vice President of R&D Jim Cassella said recently:
The issue that the FDA noted was an inspectional issue, so that was the only thing really in the letter. We addressed the issues with the CDRH reviewers who had raised the issue and then we responded to their questions. We resubmitted the NDA, which is basically a labeling resubmission, so there is not a lot of work left to do. We believe that we have addressed the inspectional issues and we have resubmitted an advanced draft of the label and we have probably a little bit of work left to do on some of the REMS documents.
Since there was no clinical or safety issues identified, and no other deficiencies outlined in the CRL, Adasuve has a good chance at approval this time in my opinion. Manufacturing, labeling, and REMS can be dealt with, efficacy and serious safety concerns cannot.
| Share Statistics | |
| Avg Vol (3 month): | 506,563 |
| Avg Vol (10 day): | 801,857 |
| Shares Outstanding: | 15.69M |
| Float: | 14.80M |
| % Held by Insiders: | 19.55% |
| % Held by Institutions: | 18.10% |
| Shares Short (as of Oct 31, 2012): | 1.96M |
| Short Ratio (as of Oct 31, 2012): | 3.70 |
| Short % of Float (as of Oct 31, 2012): | 17.10% |
| Shares Short (prior month): | 1.99M |
Alexza has a very small float of which over 17% of it is held short. An FDA approval for Adasuve would likely cause a short squeeze. It's a good bet the smarter shorts will be covering their positions as we draw close to both catalyst event dates. Again, if safety and efficacy issues were the main concern here, then I could understand shorts holding their positions. However, this is not the case.
Recently, we saw a 200% jump in Acadia Pharma's (ACAD) stock price when its drug Pimavanserin showed positive Phase III clinical results. Pimavanserin is designed for the treatment of Parkinson's disease psychosis. Adasuve is similar to Pimavanserin in that both are designed to treat psychosomatic disorders, and both drugs saw initial failures. Also, Acadia still needs to engage in another clinical study for Pimavanserin, so there still remains considerable uncertainty for the drug. Therefore, I feel Acadia is overvalued at its current price level because there is too much downside risk at this time for me to consider it a good long trade/investment.
I would not be surprised to see a double of the Alexza stock on FDA approval, should the drug be approved this time around. However, there is considerable risk for traders and investors holding through the PDUFA date. If the FDA rejects Adasuve again, it's likely Alexza as a company will not survive because they will simply run out of cash. At the very least, the company would have to engage in dilutive financing to survive. While I believe this will not occur, it's wise to consider this possibility.
The chart appears to be showing signs of making an continuation upwards after a brief period of weakness that was likely related to some profit taking. Note the RSI is trending back towards the green line and I expect it to cross it again shortly. A move back over $6 looks within range.
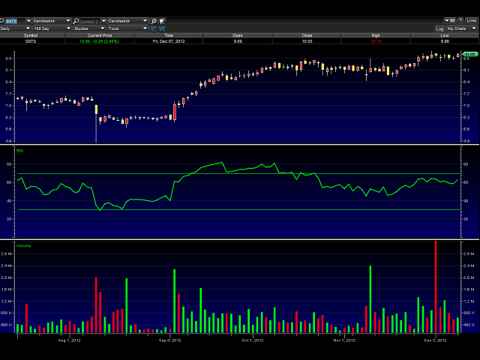
Santarus (SNTS)
Santarus engages in acquiring, developing, and commercializing proprietary products that address the needs of patients treated by physician specialists. The company sells metformin hydrochloride extended release tablets under the Glumetza name and bromocriptine mesylate tablets under the Cycloset name, which are indicated to treat type 2 diabetes. The company also sells fenofibrate tablets under the Fenoglide name that is used to treat primary hyperlipidemia or mixed dyslipidemia. Finally, omeprazole/sodium bicarbonate capsules and powder are sold for oral suspension under the Zegerid name for the treatment of upper GI conditions, including gastroesophageal reflux disease.
Moving factor: FDA Catalyst event, chart technicals.
I consider Santarus a good catalyst trade as the company is drawing closer to its Prescription Drug User Fee Act (PDUFA) target action date of January 16, 2013, for the review of its New Drug Application for Uceris (budesonide) 9 mg tablets.
Uceris is an investigational drug that contains budesonide, a corticosteroid, in a novel oral tablet formulation that utilizes proprietary MMX ® multi-matrix system technology, which is designed to result in the controlled release and distribution of budesonide throughout the length of the colon.
Both the company's Phase II and Phase III studies were statistically significant for the induction of remission of active, mild, and moderate ulcerative colitis with over 1000 patients, so it looks like approval is a good bet here.
Although it might appear that the chart is topped out, the RSI is still below the green line, and every time the RSI has approached this point, it crossed above the line, and the stock price followed. A move to around $11 in the short term looks likely in my opinion.
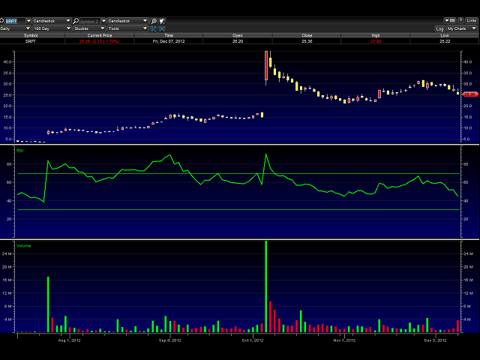
Sarepta Therapeutics (SRPT)
Sarepta focuses on the discovery and development of RNA-based therapeutics for the treatment of serious and life-threatening rare and infectious diseases. Its lead clinical candidate is Eteplirsen, which is in clinical Phase 2 for the treatment of Duchenne muscular dystrophy (DMD). The company is also developing treatments that are in Phase I clinical trials for infectious diseases including AVI-7537 for Ebola virus, AVI-7288 for Marburg virus, and AVI-7100 for influenza. Its pre-clinical products are comprised of Exon 45 PMO and Exon 50 PMO, which are used for the treatment of Duchenne muscular dystrophy. The company's discovery stage products consist of Viral PMO-X for the treatment of dengue fever and Bacterial PPMO used in the treatment of tuberculosis.
Moving Factors: Oversold chart, creating an undervalued condition.
The company released its 62-week data from its Duchenne muscular dystrophy drug Eteplirsen last Friday, and apparently the market was not as enthused as it was when the company released its 48-week look at the drug back in October. At the time, the 48 week data produced results that indicated the drug may revolutionize the way the disease is treated. It not only slowed DMD's progression, but actually lengthened the distance patients were able to walk. The 62-week results were good, but perhaps not as good as the market hoped for since the stock sold off a bit on the news.
DMD is a genetic disease that causes increasing muscle weakness. It is caused by a lack of the protein dystrophin, and it affects only very young males.
DMD occurs in about 1 of every 3,600 male infants. The National Institutes of Health say patients typically die before the age of 25, so a potential cure for this disease would be a huge breakthrough in medicine.
The weakness in the stock might be a good opportunity for a long based trade this week. Since Sarepta's stock is up 550% for the year, we might see a bit more profit taking to being this week before the stock makes a move back up. Regardless, it's my opinion that the stock currently offers a nice beginning entry point at its current level.
The green line below the actual price chart is the Relative Strength Index (RSI), which sharply turned down after Friday's sell-off. Just about every time the RSI has turned to where it is now, it rebounded and turned back upwards. I consider the recent sell-off to be a little bit over done, so this might be a very good opportunity for either a quick swing trade, and/or a nice entry point for a longer term hold.
Ariad Pharma (ARIA)
Ariad focuses on the discovery, development, and commercialization of medicines for cancer patients. Its lead product in development is Ponatinib, an investigational pan BCR-ABL inhibitor, which is nearing completion of its pivotal Phase II clinical trials in patients with hematologic cancers. These include chronic myeloid leukemia and Philadelphia positive acute lymphoblastic leukemia.
Moving factors: Near-term FDA catalyst event, chart technicals.
Ponatinib has fully enrolled its pivotal Phase II PACE (Ponatinb Ph+ ALL and CML Evaluation) trial in patients with resistant or intolerant CML and Ph+ ALL. Results from the Phase II PACE trial of Ponatinib were reported at the annual meeting of the American Society of Clinical Oncology (ASCO) and the 2012 European Hematology Association (EHA) annual congress. Ariad's Phase III EPIC trial comparing Ponatinib to imatinib is continuing for newly diagnosed chronic-phase CML patients.
Ponatinib is a BCR-ABL inhibitor, is designed for heavily pretreated patients with resistant or refractory chronic myeloid leukemia (CML), or Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL).
Over the weekend on Saturday, Ariad announced twelve-month follow-up data from the pivotal PACE trial of Ponatinib. The study showed that 56% of chronic-phase CML patients in the trial, including 70% of patients with a T315I mutation, achieved a major cytogenetic response (MCyR), the primary end-point for chronic-phase CML patients.
Ponatinib has really made a difference in the lives of some people suffering from the forms of leukemia mentioned above. This because the FDA has allowed Ariad to treat people suffering from these forms of leukemia via compassionate use, which allows certain qualified patients to try the drug outside of clinical trials.
In July 2012, Ariad submitted a New Drug Application (NDA) for Ponatinib to the FDA. Ariad is seeking U.S. marketing approval of Ponatinib in patients with resistant or intolerant CML Ph+ ALL. The company is seeking accelerated approval for Ponatinib by the FDA and has requested a priority review of the application.
Ariad expects an FDA approval decision for the drug sometime in Q1, 2013. The company's stock price has risen at a parabolic rate from around $0.45 in a little over 3 years, to a recent 52 week and all-time high of $25.39.
The chart looks very bullish to me, showing a nice continuation triangle and a strongly trending upwards RSI. $25 a share looks to be close on the horizon. Traders and investors might want to consider an entry point in the stock when and if the stock shows moments of weakness.

Hello Scott,
What your take on array? I’m still holding, however it’s going more south then north on the pps.
It’s about to head back north again in my opinion according to the chart
Nice call, I will try to sell at 4.00. Thanks again
Sorry its off your topic but I’d like to get your take on DVAX since I am holding the bag. Thanks.
I’m not wild about DVAX, but I will take another look, cool?
Your a mentch. Thanks
Hello Scott, I know you have looked at MNOV in the past, do you have any thoughts on it going forward into next year? They’ve been extending their patent protection and have clinical trials for treatment of asthma and opioid addiction (and heroine) that have potentially huge market potential in my opinion. I’m just starting my DD for MNOV.
Look forward to hearing from you! Thanks!
I’ve been trying lately to reach their IR, and they do not reply, which is always a bad sign — time to fire IR get a new one.
Thanks, Scott! We’ll have to just wait and see what happens early next year for MNOV.
P.S.: ISR – Isoray looks sweet for next year! 😛
I’ve been holding AMRN since above 12….. what is your feeling about holding it? Looks like they are going on their own, at least for a quarter or two.
I like the asset, hate the CEO, it’s going to be hard for AMRN, but it might work out in the long run.