Investment Thesis with Halozyme (HALO) and Story:
Last year HALO was a one of the high flying biotech stocks, and increased in value 5 x.
Much of Halo's big run occurred when we mentioned that a company director bought 50,000 shares in the high $6 range. This same director bought 50,000 shares of Jazz Pharma in 2009 for a price around $9 -- she is a long term investor and still owns Jazz shares. We saw this as a strong sign of insider confidence along with the fact this person had great success doing the same with Jazz.
The speculation got hotter for Halo when SC Herceptin and SC MabThera approval catalyst dates approached late last year for the European market. Both products eventually were approved in Europe. Roche pays a royalty to Halo for these two products, which really is not a significant value drive in and of itself -- but many fail to understand Halo's platform and why we believe it far more valuable than many biotech investors realize.
In December 2006, Halozyme entered into an agreement with Roche to apply Halozyme's patented Enhanze™ technology (recombinant human hyaluronidase or rHuPH20) to Roche's biological therapeutic compounds. To date, Roche has elected to explore the use of rHuPH20 for up to a total of five exclusive targets.
Enhanze technology uses a proprietary enzyme to facilitate the absorption and dispersion of drugs or fluids that are injected under the skin. When injected under the skin, the enzyme transiently generates channels in tissues underlying the outer layers of the skin to increase the absorption and spread of injected drugs.
Halo's lead enzyme, the recombinant human hyaluronidase (rHuPH20), temporarily degrades hyaluronan, a matrix component in the skin, and facilitates the dispersion and absorption of drugs and fluids.
Let's see how Halozyme describes its' platform:
"ENHANZE™ Technology is a proprietary delivery platform using our first approved enzyme, recombinant human hyaluronidase – rHuPH20. This enzyme temporarily degrades hyaluronan, a structural component of the subcutaneous space that is just beneath the outside surface of the human skin. This temporary degradation creates an opportunistic window for the improved subcutaneous delivery of injectable biologics such as monoclonal antibodies and other large therapeutic molecules, as well as small molecules and fluids. With the hyaluronan temporarily degraded, molecules as large as 200 nanometers may pass freely through the subcutaneous space.
The hyaluronan reconstitutes its normal density within several days and, therefore, any effect of the rHuPH20 on the architecture of the subcutaneous space is temporary. By using Halozyme’s rHuPH20 enzyme, many therapeutics that could normally only be injected intravenously (IV) can now be administered subcutaneously (SC). This change in the route of delivery to SC from IV can often improve patient convenience, enhance used pharmacokinetics, boost efficacy, extend the product lifecycle and reduce cost.
Halozyme currently has four ENHANZE™ Technology partnerships, including one with Hoffman-La Roche to apply the technology to up to eight targets including Hoffman-La Roche’s biological therapeutics Herceptin® and MABTHERA® (both of which are approved in Europe for the SC formulations of their products using rHuPH20). Halozyme has four additional partnerships with Baxter Healthcare for immunoglobulin and Pfizer."
In a nutshell, Enhanze is not some new drug, but a system designed to make existing drugs more potent via faster and more direct delivery. So, Halo can partner with an unlimited amount of companies and their respective drugs. Hence the word "Enhanze" as in "Enhance." With the ability to enhance a variety of drugs via subcutaneous injections, Halo can announce at any time a new partnership with just about any pharma, and has the ability to announce several in any given year. This is important because this adds PPS VALUE each time an event like this occurs, allowing investors and traders to decide to "sell into up gaps" if they so choose.
- Catalyst - Binary Event - Adcom coming up July 31, 2014
Halo and its royalty partner Baxter Pharma are expecting an Adcom on July 31 for its Biologics License Application (BLA) for HYQVIA (Human Immune Globulin Infusion 10% with Recombinant Human Hyaluronidase), the company’s investigational subcutaneous treatment for patients with primary immunodeficiency (PI) .
The Adcom will give guidance to the FDA on whether the BLA is acceptable in its new amended form. In other words, they will decide whether or not to recommend HyQvia to the FDA for approval or not.
HyQvia is a combination of human immune globulin (Baxter's drug being enhanced) and recombinant human hyaluronidase (Halo's Enhaze enhancer). Baxter and Halozyme submitted additional preclinical data that was requested from the FDA in 2012, and amended their collective biologics license application (BLA).
Human Immune Globulin is a substance made from human blood plasma. The plasma, processed from donated human blood, contains antibodies that protect the body against various diseases.
The FDA did not issue a CRL in 2012, but rather requested that both companies submit additional data as part of an amended BLA. This is part of the PDUFA that the FDA will issue a decision within 30 days on after it receives input from the Adcom on July 31. In a nutshell, the FDA really does not understand (like many) the how's and why's of HyQvia, and needs input from those who likely do, the Adcom panel.
Since after the FDA asked for more information, Halo has focused more on getting its platform approved in Europe. As mentioned above, it has concluded this with its partner Roche, while Baxter has been taking the lead with the Amended BLA.
- Adcom Positive recommendation not a slam dunk, but we are optimistic.
In 2012, ViroPharma (VPHM) was using Halo's platform to develop a subcutaneous version of VPHM'S Cinryze.
ViroPharma announced that it was discontinuing the overall development of subcutaneous Cinryze with Halozyme's rHuPH20 technology after Phase II trial after the trial was observing an "unexpected increase and titer of non-neutralizing anti-rHuPH20 antibodies" However, there were no adverse effects from the increase in those antibodies. ViroPharma however was developing its own type of "enhancer" as a back up, and has since gone to this back-up to move its subcutaneous Cinryze forward -- without Halo.
The above is what the "bears" will point to to suggest the Adcom, and subsequent approval chances for HyQvia will result in a negative outcome. Although it's something to consider as a risk, we point out that Cinryze (C1 esterase inhibitor [human]) is an injectable prescription medicine that is used to help prevent swelling and/or painful attacks in teenagers and adults with Hereditary Angioedema (HAE). Each biologic has its own matrix, and to the best of our and the European Medicine's Agency's (EMA) knowledge, there are no known issues that were considered unsafe since it has already approved this product for use in Europe. If there were safety issues, the EMA would have rejected HyQvia.
We feel this is important having HyQvia already approved for use in Europe. In 2012, it was not yet approved in Europe. Halo and Baxter will certainly point this fact out at Adcom, and is likely to use notes from the EMA approval decision if needed.
Therefore, it's my opinion/our opinion that a positive outcome from Adcom is about 80%. If the Adcom is positive, it's about a 99% chance that the FDA will approve.
- PEGPH20 (PEG) - Halo's potential blockbuster that if approved, could potentially revolutionize certain cancer treatments.
Halo's Website offers the following explanation of what PEG is and how it works;
"Certain cancers, including pancreatic, breast, colon and prostate, have been shown to accumulate high levels of hyaluronan (HA). Aberrant accumulation of this component of the tumor’s infrastructure supports a protective network or “halo” that surrounds certain tumors. This pathologic accumulation of HA along with other matrix components also increases tumor interstitial fluid pressure, constricting tumor vasculature and creating a unique microenvironment for the growth of tumor cells compared to normal cells.
These mechanisms generate barriers to drug delivery that inhibit the potential effectiveness of many anti-cancer agents. Dismantling the HA component of the tumor architecture disrupts this unique tumor microenvironment and opens the previously constricted vessels which may increase blood flow to the tumor. This may allow cancer therapies to be more efficiently delivered to their target and thus may be more effective.
Halozyme’s FDA-approved, HYLENEX® recombinant human hyaluronidase, rHuPH20, is administered subcutaneously and temporarily and reversibly degrades HA to facilitate the absorption and dispersion of other injected drugs or fluids and for subcutaneous fluid administration. However, rHuPH20 acts only locally at the injection site, is rapidly inactivated in the body, and does not survive in the blood. An investigational PEGylated form of rHuPH20, PEGPH20, has been developed by Halozyme which dramatically increases the half-life of the compound in the blood and allows for intravenous administration."
Essentially, PEG is a PEGylated form of the company's Enhaze technology, specifically known as HYLENEX (rHuPH20). rHuPH20 has a very short lifespan in the blood, and the time it takes the blood to reach the HA area of a tumor, there would be zero level of any particular drug designed to treat the tumor. RHuPH20 only acts at the injection site, but PEG has been designed to go beyond this and penetrate the HA of a deep malignant tumor, like one that could exist in the pancreas.
Pancreatic cancer, unless caught extremely early is basically a death sentence which I have personally witnessed the vile effects of with a close family friend years ago. If this treatment is proven to work, and the risks are tolerable, Halo would likely be worth 10's of billions of dollars.
However, before we get excited here, PEG has a long ways to go to actually prove it's safe and ultimately effective. Recently, the ongoing trial in combination with gemcitabine and Abraxane used in conjunction with PEG was first halted by the company voluntarily and then the FDA after discovering a potential safety issue. This issue was related to an imbalance in the thromboembolic (blood clots) event rate between the PEG-PH20 arm and the control arm of a Phase II trial in combination with the a fore mentioned drugs, gemcitabine and Abraxane.
However, both the company, then later the FDA announced that the trial would continue. Regardless, investors need to keep a close eye on this trial for something like this potentially popping up again. The reason is rather simple:
What happened recently with PEG was that using a slightly different, more direct and potent form of Enhanze, it will stay and travel in the blood stream much longer in order to penetrate the HA of a deep tumor. With the longer half life in this version, it has the potential to thicken the blood to dangerous levels of clotting, especially near the injection site, then traveling further into a patients blood stream.
If Halo can show these events can be limited, the FDA would likely accept the risk/reward profile here because of the cancers PEG will target -- pancreatic, breast, colon and prostate.
- Risk/reward of PEG and where it's likely acceptable
As mentioned prior, pancreatic cancer is a basic death sentence, and it's my opinion the FDA lifted the halt of the trial in question because it knows that to be the case. This lift is an early indicator to me that if PEG has the efficacy which I believe it has, there is a good chance at approval for use with gemcitabine and Abraxane to treat pancreatic cancer which would equate to potentially billions in revenues. This combination would very likely be a front-line treatment for mid to later stage pancreatic cancer because again, it's so deadly and a wide open unmet need.
In later stage breast cancer, the blood clot potential risk would likely be accepted, but not in the mid or early stages of this particular disease. So PEG, in conjunction with other treatments, would likely be a 3rd or last line treatment option.
Prostate - 3rd line treatment option
Colon - potentially 2nd line treatment option.
Any such approval for its first line and hence most lucrative revenue producer, pancreatic cancer, should arrive somewhere in 2018 with subsequent NDA's for the other cancers mentioned above by 2025.
Therefore, while certainly a risky speculation investment, its longer term potential if everything goes decently could see a stock price based on the current number of outstanding shares upwards over $300 -- a Huge reward. This is not a penny stock "get rich" proposition. There is a very realistic chance that in time, Halo could be a goldmine.
However, if you so choose to make an investment here, please remember that this will take time and a lot of patience -- please consider all the risks involved. Additionally, we feel that Halo in the short term could see a price approaching $11 a share because of the renewed positive sentiment and good chance of positive Adcom for Hyqvia at the end of July -- immediately before the Adcom notes are released, normally 2 to 3 business days in advance of the actual Adcom panel meeting.
- Management Rating And Trust + Randall Kirk as a large investor.
To understand Kirk a bit, he is generally a finance guy and not really so tech savoy. While it's important and positive he is on board here, his investment will have no effect on the future success of Halo, and especially if PEG will prove effective and have acceptable safety risks, or not.
Helen Torley , M.B. Ch. B., M.R.C.P. President, Chief Executive Officer (CEO) and member of the Board of Directors.
Dr. Torley served as Executive Vice President and Chief Commercial Officer for Onyx Pharmaceuticals (Onyx) from August 2011 to December 2013, overseeing the collaboration with Bayer on Nexavar® and Stivarga® and the U.S. launch of Kyprolis.
Many believe Torley was a chief facilitator in the deal that saw ONYX acquired by Amgen (AMGN) for $10.8b in September of last year.
I speculate that Torley has been brought on board to help facilitate a deal to sell Halo in the future, which would likely happen after PEG is further along in the clinical process. An acquisition of Halo right now would present a problem for an acquirer because they would basically have to honor the royalty deals in place, which may or may not include the deals with Roche and others. There could be language in the royalty agreements that gives those companies, "right of first refusal," which basically means any of these companies can block a sale of the ASSETS for which they are partnered in, but not PEG, which is wholly owned by Halo at this time.
Torly did a fine job at Onyx, and is is a more than competent operator. CEO rating - 7.5 out of 10.
Kirk has also been know to facilitate acquisitions, and Torley may have been brought aboard here to eventually do just that -- sell the company.
Any such acquisition of Halo would strictly be for the PEG asset. Therefore, a big pharma is going to want to see how the clinical process progresses with PEG.
Board of Directors member Kathryn E. Falberg:
As mentioned above and written on by us a few times, Falberg purchased 50k share of Halo around $6.80 a share last year. We found this to be highly significant as she also purchased the same 50k shares of Jazz Pharma for around $9 in 2009., where she was also a board member. Her investment in Jazz has obviously paid off in a huge way. She has the same conviction with Halo and has a good track record, so this is of note in terms of her putting up her own money without simply being awarded those shares via options.
| Income Statement | |
| Revenue (ttm): | 54.93M |
| Revenue Per Share (ttm): | 0.48 |
| Qtrly Revenue Growth (yoy): | 1.10% |
| Gross Profit (ttm): | 48.55M |
| EBITDA (ttm): | -85.83M |
| Net Income Avl to Common (ttm): | -90.74M |
| Diluted EPS (ttm): | -0.79 |
| Qtrly Earnings Growth (yoy): | N/A |
| ||||||||||||||
| ||||||
Halo burns about $14.5M a quarter and this could increase as PEG ramps up, but can also decrease for reasons I mention below. Nonetheless, investors should expect secondary public offerings from time to time with Halo. This is a short term PPS risk as far as trading the stock for a catalyst run-up from now until July 31. However, it's not a hugely significant risk as Halo has approx. twelve quarters of operating cash, and a manageable debt ratio. However, investors need to keep an eye on the - $34.43M levered free cash flow and total debt number which stands at about $50M now to gauge how management handles its finances. It should also be noted that as its products ramp up in Europe for which they are approved, and if the FDA approves Hyqvia sometime in Late August/early September, the burn rate could drop significantly, easing capital raise concerns/dilution on investors.
| Valuation Measures |
|---|
| Market Cap (intraday): | 1.06B |
| Enterprise Value (Jun 7, 2014): | 824.95M |
Halo's current market cap of $1.06B is not reflecting what I feel many investors are missing in terms of the 1st line potential of PEG helping to treat pancreatic cancer. As mentioned prior, the FDA allowing this trial to move forward indicates to me they clearly understand just how deadly mid and late stage pancreatic cancer is. It also signals that the blood clots risks might end up as acceptable, unless these events have the potential to affect every patient which is not the case so far. However, because of the recent regulatory setback, it's reasonable to assume investors are being cautious here. But, because of the upcoming catalyst, the pps should run up significantly because of its good chance at a positive adcom and the current excessive liquidity speculation in the markets.
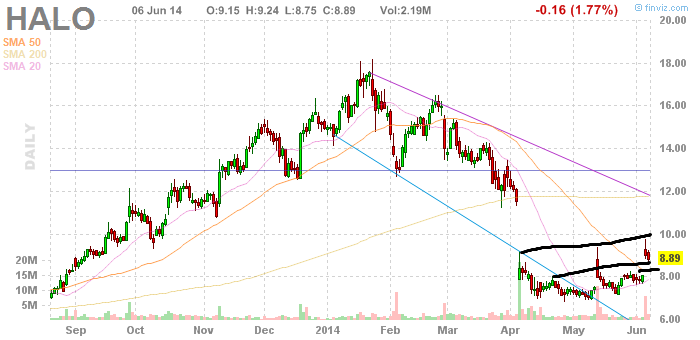
Chart:
I have marked in crude black lines to show areas of support and resistance. The top line is the area of resistance which has been slowly trending upwards. The gap up here can be largely ignored because of the prior large gap down and trading that occurred within the gap range before as we can see from the first green line in early April. The middle line shows where I see support for Halo, which is in the range of $8.65 to $8.85, with an up trending resistance top line of about $9.75. The last black line I made shows where a stop should be set in my opinion for those who might be planning a swing trade with Halo. This line is somewhere in the vicinity of $8.30 to $8.40.
- Risks
While the technology platform has been validated by the FDA with the approval of Hylenex and the approval of multiple products in Europe, the emergence of new adverse events associated with the technology could arise and weigh on the shares. Also, as Halo increases its clinical trial activity, it may need to return to the capital markets for additional funding which would dilute existing shareholders. But, this could be offset by the royalty payments it receives from Roche with products approved in Europe along with payments from Baxter upon FDA approval of HyQvia.
Additionally, The PEG Pancreatic cancer trial could see more blood clotting events occur that could become excessive. While I feel this is not likely after the FDA lift of the P2 trial halt, it should be mentioned as a risk to consider which falls under regulatory concerns.
- Rewards
Potential for a continuous news flow of partnerships for its Enhaze rHuPH20 tech would likely cause pps spikes and good opportunity for investors and traders to take healthy profits.
Potential for an early acquisition:
A surprise acquisition sometime in the next year is not entirely out of the question. If the company feels the risk/reward profile is in their favor and an acceptable offer materializes, it could be considered wise to sell -- we saw this with Trius last year -- PEG success is not by any means a guarantee.
However, an acquisition in the shorter term could prove to be a very difficult proposition if Roche, Baxter and others have "right of first refusal clauses" in their respective deals with Halo. This situation could be worked out, but it would be a messy one. This is assuming there is in fact a right of first refusal clause in any and all of these deals.
Finally, the biggest reward is if PEG eventually is FDA approved for the pancreatic cancer indication. Without a doubt, it would be a first line treatment in basically what is a wide open unmet need area - mid to late state pancreatic cancer.
If this occurs and the company is not acquired, it's very possible and even likely a pps of $300+ can be obtained based on the current outstanding shares. Any such FDA approval would come no earlier than 2018, and possibly 2019.
- Price Target opinions
In the short term, running up to Adcom on July 31, It's my opinion that Halo reaches the $11.50 range, assuming decent market conditions. Short term trade rating = 7 out of 10
The mid term is uncertain as any regulatory set back would put pressure on the shares, and we could see a lot of volatility in the stock -- as we saw last year and into this year with Halo.
We could see Halo gain stronger speculation as new royalty deals are announced that could take the stock into the low $30 range and/or bumps in the road that get us back down to the current level under $9. I think of $CLDX as an example of volatile pps movement over the last year -- we could see similar with Halo.
It really all depends on continued regulatory success with Halo. I lean about 65/35 to the positive/upside here as I believe its regulatory issues are mostly behind the company. Mid term trade rating - 6.5 out of 10
Long term price target could see in excess of $300 a share if PEG wins approval in 4 years or so based on the current share count of around 120M. Long term (2 years +) speculative investment rating - 9.5 out of 10
Please remember, if you are going to trade this one as a trader, then approach it as a trader and use stops!
If you choose to make an investment, understand that there is significant risk here. While I am bullish on Halo's long term chances, as with any biotech, unexpected set backs can occur (recent PEG halt as an example), and PEG success is not assured by any means.
Please be sure to do as much due diligence as humanely possible when considering a significant long term investment.
Feedback of our new way of issuing reports is most welcome. I hope you have found this report informative and helpful!
Scott

Awesome and very helpful report Scott, thanks.
more to come, you are welcome!